The Novo Nordisk company, taking social responsibility and caring for patients, informs about the current situation with illegal import and trafficking of medicines such as Ozempic®, Saxenda® and Rybelsus® on the territory of the Republic of Kazakhstan.
Novo Nordisk became aware of the fact of illegal import, as well as the dissemination of false information and discrediting of the company's medicines, through social networks: Instagram and Telegram.
Novo Nordisk does not recommend purchasing medicinal products, in particular Ozempic®, manufactured for sale on the markets of other countries.
Ozempic® has special storage and transport conditions, and the company cannot guarantee the quality and safety of illegally imported preparations. By purchasing such products, you are at risk of encountering a low-quality product and bearfull responsibility for the potential safety risk.
Our company takes appropriate actions to prevent cases of illegal import of Novo Nordisk medicines:
- Collect data on illegal import cases, timely inform global security teams, crack down on illegal online sales, remove social media/marketplaces/websites.
We would like to remind you that according to the legislation: Only an official distributor licensed for the wholesale distribution of medicines, has the right to legally sell Novo Nordisk products through pharmacy chains in the territory of the Republic of Kazakhstan, subject to compliance with all transport and storage standards set by the company.
Be aware of the risk of counterfeit products and carefully check the purchased product before use.
If you find a counterfeit product, do not use it and contact by
email
eaeu-safety@novonordisk.com or by phone +7 (727)
3307788. If you have used a product that you suspect is counterfeit,
contact your doctor immediately.
Patient safety is a top priority for Novo Nordisk and we are in close dialogue with all stakeholders to best support patients in the fight against counterfeit products.
If you have any questions, please contact us by email at eaeu-safety@novonordisk.com, or by phone at +7 (727) 3307788.
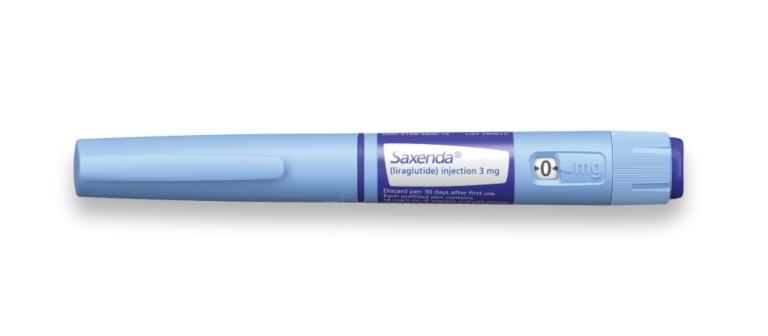
Novo Nordisk has been made aware of some Ozempic® and Saxenda® pens in Uzbekistan, Azerbaijan and Kazakhstan that have been falsified and are not legitimate. The falsified products have been sold both within the legal and illegal supply chain. The content of the falsified pens is entirely different from the genuine products and should not be used as the falsified pens pose a risk to patients’ safety.
The main difference between the counterfeit product and the genuine product is that the genuine Novo Nordisk Ozempic® and Saxenda® pens do not extend or increase in length when setting the dose. The scale drum increments are fixed doses, such as 0.25 mg, 0.5 mg, and 1.0 mg for Ozempic® and 0.6 mg, 1.2 mg, 1.8 mg, 2.4 mg and 3.0 mg for Saxenda®.
A falsified pen can be identified based on scale going from 0 to 80 units and extending out from the pen when setting the dose.



Patient safety is a priority for Novo Nordisk.
In case of a
side effect, please contact
eaeu-safety@novonordisk.com
or by phone +7 (727) 330 77 88.
You can also contact us in the
Contact Us section.
To access your profile outside of the application process you will need to select a position on the website and click Apply now. Once you click Apply now you will be routed to the position page where you can select View Profile from the top menu bar, sign in and open the Jobs Applied accordion.
After you sign in you will be able to view the position(s) to which you have applied, view your application(s) and the status of your application(s).
After your log into your profile you will be taken to the candidate profile screen where you can select My Documents.
Here you can upload from a device, drop box or google drive. Note, you can only maintain one resume on file at a time.
Talent Acquisition will review your resume and interest in conjunction with the requirements of the role. If there is interest in having you move forward in the process you will be contacted by a Novo Nordisk Talent Acquisition Representative regarding next steps.
As a reminder, you can always view the status of your application by navigating to View Profile and selecting Jobs Applied.
You can always view your application status once you log into the
system and select View Profile and by selecting Jobs
Applied.

